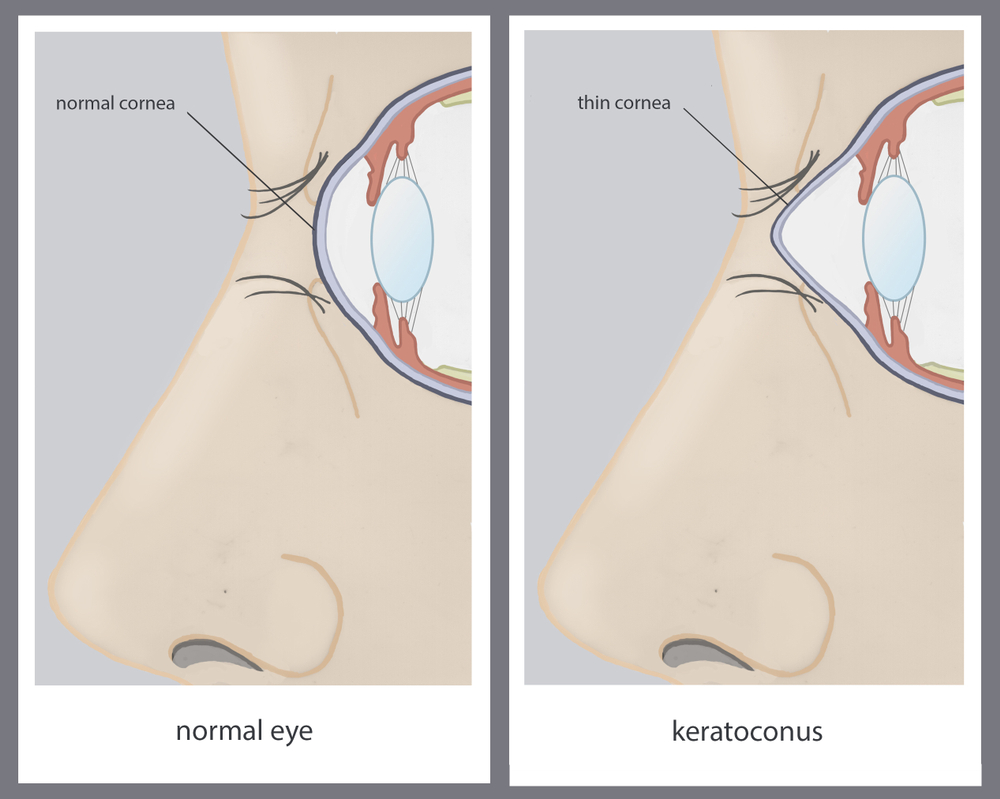
Keratoconus and other corneal ectatic disorders can significantly impact your vision and quality of life. Fortunately, advancements in ophthalmology have introduced effective treatments, like the iLink™ procedure, a minimally invasive form of corneal cross-linking (CXL) designed to halt the progression of these conditions. If you’ve been diagnosed with keratoconus, understanding the iLink™ procedure can help you make an informed decision about preserving your vision.
What Is iLink™ Corneal Cross-Linking?
iLink™ is the only FDA-approved cross-linking treatment for progressive keratoconus. It’s designed to strengthen the cornea, the clear, dome-shaped surface of your eye, by creating new collagen bonds within the corneal tissue. This procedure not only stops keratoconus from worsening but can also enhance corneal stability. The iLink™ procedure is considered safe and effective, offering long-term benefits for individuals diagnosed with progressive keratoconus.
How Does iLink™ Work?
The iLink™ procedure combines ultraviolet (UV) light and riboflavin (vitamin B2) to reinforce the corneal structure, effectively halting the progression of keratoconus. The process is straightforward and involves four key steps.
The first step is preparation. Your ophthalmologist will numb your eye using anesthetic drops to ensure comfort. They will then remove a thin layer of the corneal epithelium, allowing better penetration of riboflavin into the corneal tissue.
Next is the riboflavin application. Riboflavin drops are applied to the cornea at regular intervals over approximately 30 minutes. This process ensures the cornea absorbs the vitamin adequately, preparing it to respond to the UV light used in the next step.
The third step involves UV light exposure. Once the riboflavin has been absorbed, the cornea is exposed to UV light for about 30 minutes. This exposure activates the riboflavin, initiating a chemical reaction that strengthens the collagen fibers and stabilizes the cornea.
Finally, the procedure concludes with post-treatment care. A soft contact lens is placed over the treated eye to encourage healing. Your doctor will also provide eye drops to prevent infection and reduce inflammation, ensuring a smooth recovery process.
Benefits of iLink™ Corneal Cross-Linking
Stops Progression: The primary goal of iLink™ is to halt the progression of keratoconus, preventing further vision loss.
Improves Corneal Strength: By reinforcing the corneal collagen network, the procedure stabilizes the eye’s structure.
Minimally Invasive: iLink™ is a quick, outpatient procedure with minimal discomfort and downtime.
FDA-Approved: iLink™ is the only cross-linking treatment approved by the FDA, ensuring its safety and efficacy.
What to Expect During Recovery
After the iLink™ procedure, it's normal to experience mild discomfort, light sensitivity, and blurry vision for a few days. Complete healing of the corneal epithelium may take 5-7 days, during which your doctor will monitor your progress and adjust your treatment plan as necessary.
Follow-up appointments are crucial to ensure proper healing and assess the effectiveness of the procedure. Your ophthalmologist will also guide you on when to resume your daily activities.
Is iLink™ Right for You?
The iLink™ procedure is typically recommended for patients with progressive keratoconus, especially in its early stages. If left untreated, keratoconus can lead to significant visual impairment and may require more invasive interventions like corneal transplantation.
However, the iLink™ procedure is not suitable for everyone. A thorough evaluation of your corneal thickness, condition, and overall eye health will determine your candidacy.
Get in Touch with Coastal Vision Medical Group Today
The iLink™ procedure represents a groundbreaking advancement in the treatment of progressive keratoconus, offering a safe and effective way to halt its progression and stabilize your vision. With its FDA approval and minimally invasive approach, iLink™ gives patients a chance to protect their eyesight and maintain their quality of life.







